- Sponsored / 9 Min Read
There’s No Doubt About It, Early Detection Saves Lives…
And This Molecular Genetics Diagnostic Company May Just Have The Answer To Save Countless Lives Worldwide.
Here’s how Mainz BioMed could take full advantage of a $3.7 BILLION market opportunity with its blockbuster early detection test for Colorectal Cancer. (1)
📰 Breaking News | April 26, 2022
Mainz Biomed - 4 Key Reasons To Get This Potentially Undervalued Idea On Your Radar ASAP
01
The Company’s Flagship Product (ColoAlert) Could Become An Inexpensive Alternative In A $3.7 Billion Market
02
03
04
The chances of it impacting either yourself, your family, or your friends appears to be growing on a yearly basis.
Though types like breast, lung, and prostate cancer are typically the most talked about, they’re not the only forms out there.
What do you know about colorectal cancer? Did you know it is the 2nd most lethal form of cancer in the United States? (2)
Good news is, it is highly preventable with early detection providing 5- year survival rates above 90%. (2)
How Does Colorectal Cancer Develop?
Colorectal cancer often develops from polyps.
These are initially benign cell clusters that occur in many people. However, the cells of the polyps can undergo genetic changes, causing the cells to mutate and become precursors of colorectal cancer called adenomas. (3)
The classification of colorectal cancer stages is often based on the so-called Dukes Stages:

Which Screening Options Are Available?

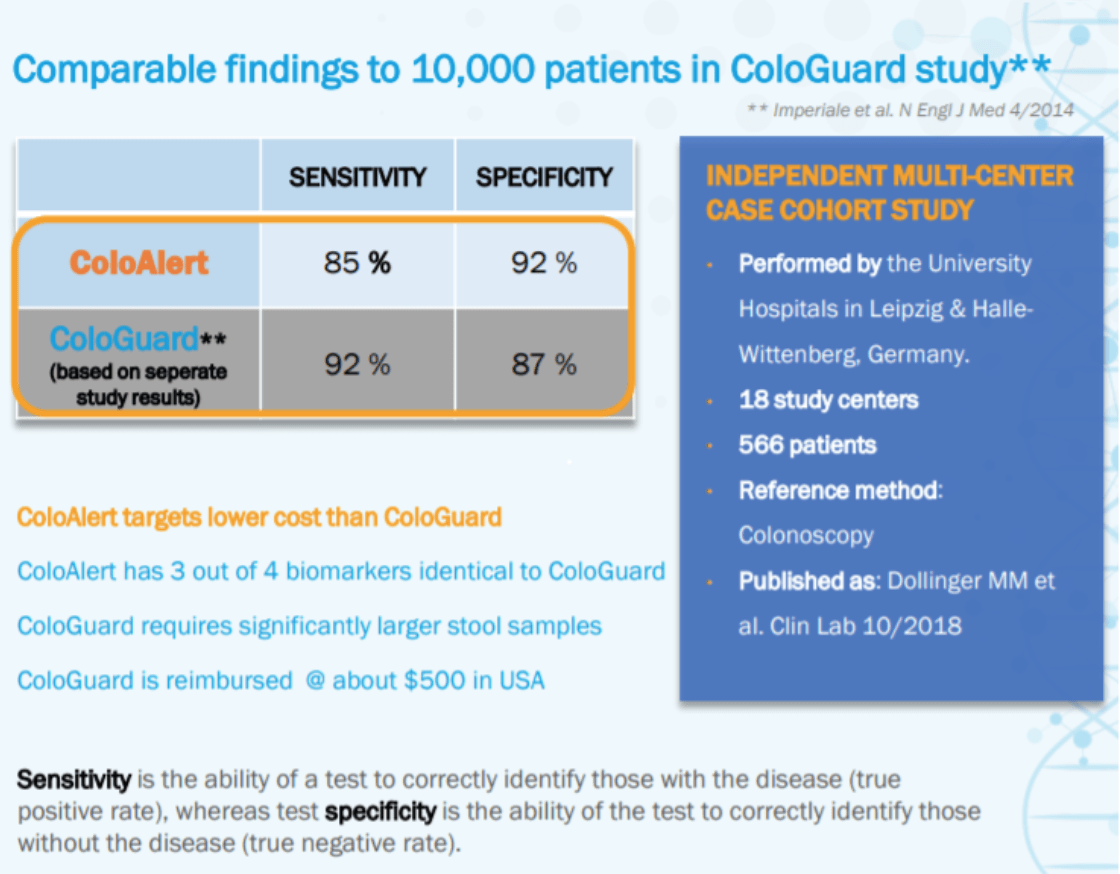
ColoAlert, Mainz Biomed's flagship product, combines the advantages of both methods.

Simple, Fast, Accurate and Non-Invasive
- A PCR-based CRC early detection stool test
- Up to 60% fewer missed cases compared to fecal immunochemical test (FIT) (1)
- Non-invasive, no preparation or sedation, no time off work
- 98% patient satisfaction – Easy product to use (1)
- Designed to offer affordable CRC screening solutions
ColoAlert Market Opportunity
Currently, there are 112 Million Americans aged 50+, a total that is expected to increase to 157 million within 10 years. (4)
- 37 Million Tests per year in the US estimated potential: 112M pop. ÷ 3 (years) @100% compliance
- 52 Million Tests per year within 10 years estimated potential: 157M pop. ÷ 3 (years) @100% compliance
- 19 Million Colonoscopies each year in USA (5)
- ~38.8% Age 50 to 75 have never been screened (in USA) (6)
- $3.7 Billion Market Opportunity (1)
Mainz Biomed: Strategic Rollout (1)
THE COMPETITION’S APPROACH…
The largest U.S. provider independently markets and distributes its product directly. All nationwide samples must come from, and be returned to, a single corporate laboratory – a time-consuming process.
MAINZ BIOMED’s APPROACH…
Mainz BioMed intends to develop and leverage scalable dissemination through a collaborative partner program.
- PARTNERSHIPS: Large lab chains incentivized to support sales & marketing efforts to physician clients & consumers.
- RELATIONSHIPS: Established regional and national labs offer existing client relationships (i.e.: physicians, clinics, hospitals, universities, government institutions, health organizations, etc).
- PROFITABILITY: ColoAlert is designed for profitability, rapid commercial uptake, and broad consumer acceptance.
- PROTECTION: Mainz BioMed protects its intellectual property through trade secrets to control all critical reagents, processes and formulations.

Why Mainz Biomed May Have Outrageous Potential
Other sector companies promote testing for CRC but are using standard tests like FIT or occult blood testing.
Currently, Exact Sciences (Nasdaq: EXAS) has a market cap of ~$14.4 billion (as of 2/10/22). (7)
Game-Changing 2022 Announcement: Mainz Biomed Acquires Exclusive Rights to Novel mRNA Biomarkers (8)
Biomarkers Demonstrated Unique Ability to Identify Curable Precancerous Colonic Polyps as well as Curable Early-Stage Colorectal Cancer
BERKELEY, Calif. and MAINZ, Germany, Jan. 05, 2022 (GLOBE NEWSWIRE) — Mainz Biomed N.V. (NASDAQ:MYNZ) (“Mainz Biomed” or the “Company”), a molecular genetics diagnostic company specializing in the early detection of cancer, announced today it has entered into a Technology Rights Agreement with Socpra Sciences Santé Et Humaines S.E.C. (“TTS”) to access a portfolio of novel mRNA biomarkers for potential future integration into ColoAlert, the Company’s highly efficacious, and easy-to-use detection test for colorectal cancer (“CRC”). Mainz is currently marketing ColoAlert in Europe through its unique business model of partnering with third-party laboratories for test kit processing versus the traditional methodology of operating a single facility. The Company is also preparing to initiate ColoAlert’s regulatory pathway for approval in the United States.
Under the terms of the Technology Rights Agreement, the Company has the unilateral option to license the exclusive global rights to five gene expression biomarkers which have demonstrated a high degree of effectiveness in detecting CRC lesions including advanced adenomas (“AA”), a type of pre-cancerous polyp often attributed to this deadly disease. In a study evaluating these biomarkers published in the online peer review journal platform MDPI (March 11, 2021), study results achieved overall sensitivities of 75% for AA and 95% for CRC, respectively, for a 96% specificity outcome. If these statistical results are duplicated when the biomarkers are integrated into ColoAlert, we believe that it will ultimately position the Company’s CRC test to be the most robust and accurate at-home diagnostic screening test on the market. It will not only detect cancerous polyps with a high degree of accuracy but has the potential to prevent CRC through early detection of precancerous adenomas.
“Securing the exclusive rights to license this family of novel biomarkers is a fantastic milestone for the Company as it provides an extraordinary opportunity to potentially upgrade ColoAlert’s technical profile, possibly making it the most effective at-home screening test for CRC that has ever been commercialized,” commented Guido Baechler, Chief Executive Officer of Mainz Biomed. “The Mainz team is on a mission to develop gold standard molecular diagnostic screening solutions for cancer indications and obtaining the rights to these biomarkers is a testament to our on-going commitment to develop cutting-edge products as they have shown superior sensitivity to even liquid biopsy products in development in terms of identifying advanced adenomas.”
And Here’s Why That Last Sentence Is So Important…
If the FDA grants premarket approval for ColoAlert, it could become a significantly cheaper alternative to ColoGuard, the product from Exact Science (~$14 Billion market cap).
Another Market Opportunity For Mainz Biomed ? You Better Believe It (1)
Mainz BioMed is currently developing proprietary genetic testing methods for pancreatic cancer.
- Fighting what could soon become the world’s second most deadly cancer. (9)
- Convenient stool test for at-home use.
- Potential for over 50 million tests per year in Europe alone.
- Supported by federal grant from Germany’s Federal Ministry for Education and Research.
- Cost of goods sold (COGS) & reimbursement analogous to ColoAlert program.
Long story short, the future potential for Mainz Biomed (Nasdaq:MYNZ) cannot be underestimated.
Mainz Biomed Recap - 4 Key Reasons To Get This Potentially Undervalued Idea On Your Radar
01
The Company’s Flagship Product (ColoAlert) Could Become An Inexpensive Alternative In A $3.7 Billion Market
02
03
04
Source 1: https://mainzbiomed.com/wp-content/uploads/2021/07/Mainz-BioMed-Corporate-Update-January-2022-v1.13.21-1.pdf
Source 2: https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html
Source 3: https://www.coloalert.com/pages/about-crc
Source 4: https://www.prb.org/resources/u-s-population-is-growing-older/
Source 5: https://idataresearch.com/an-astounding-19-million-colonoscopies-are-performed-annually-in-the-united-states/
Source 6: https://idataresearch.com/an-astounding-19-million-colonoscopies-are-performed-annually-in-the-united-states/
Source 7: https://finance.yahoo.com/quote/EXAS?p=EXAS&.tsrc=fin-srch
Source 8: https://finance.yahoo.com/news/mainz-biomed-acquires-exclusive-rights-080100033.html
Source 9: https://www.pancan.org/press-releases/pancreatic-cancer-still-on-path-to-become-second-leading-cause-of-cancer-related-death-in-u-s-by-2020/
Source 10:
https://www.mayoclinic.org/diseases-conditions/colon-cancer/in-depth/colon-cancer-screening/art-20046825